Benefits & Disadvantages of the Advanced Oxidation Process
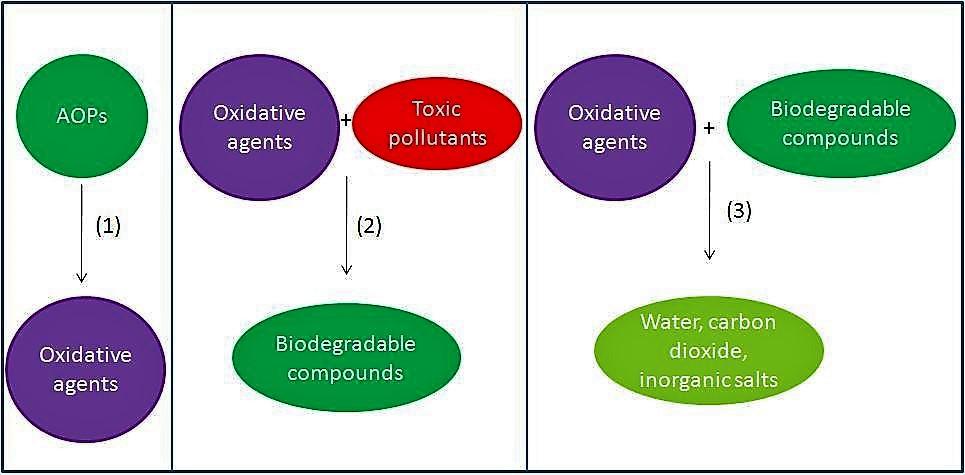
In many water and wastewater treatment applications, there are a number of pollutants that are difficult to reduce by physical, chemical, or biological means alone. In more recent years, there has been a growing concern regarding pharmaceutical drugs in drinking water and aquatic environments. Pesticides get caught in runoff from farms into freshwater supplies. Personal care products are typically washed down the drain into whatever system they are linked to. Landfill leachate is a toxic cocktail of compounds that can leak into groundwater sources. Such contaminants fall into the category of micropollutants, because they are so small. Their size alone is part of the reason, they are so difficult to remove from water and wastewater by certain means. More efficient removal requires a more powerful oxidation process, this process is called an advanced oxidation process (AOP).
This process creates powerful oxidizing agents in the form of hydroxide (OH–), but more specifically, its neutral variant the hydroxyl radical (⦁OH). Its oxidation potential is twice that of chlorine, a commonly used disinfectant. Hydroxyl radicals are the driving forces behind many advanced oxidation processes. Ozone (O3), hydrogen peroxide (H2O2), and ultraviolet light (UV) are often used in various combinations to produce ⦁OH in sufficient quantities to degrade organic (and some inorganic) pollutants. This process can reduce these pollutant concentrations, potentially from hundreds of parts per million (ppm) to just a few parts per billion (ppb).
These radicals are non-selective, therefore, they attack almost all organic materials. After these contaminants are broken down once by the ⦁OH radical they form intermediates. Those intermediates themselves react with the oxidants and mineralize into stable compounds.
Advanced oxidation has been around for several years. Therefore, this process has more than proved its usefulness, however, it is still being researched and optimized accordingly.
A powerful treatment process like the advanced oxidation process has many benefits, but it also has its share of disadvantages.
Here are just a few of the pros and cons of this particular process:
Pros
Rapid reaction rates
The OH molecule has some of the fastest reaction rates of all of the oxidants used to treat water and wastewater due to its high oxidation potentials and their non-selective nature. These quick reactions result in much lower retention times than other conventional treatment processes.
Small footprint
Because of the oxidation power of the ⦁OH radical, Advanced oxidation process units do not require much land area to process the needed flow rate for the system.
Theoretically, do not introduce new hazardous substances into water
One of the issues with chlorine disinfection is the highly toxic byproducts (DPB’s) that can result after treatment. To prevent these byproducts, an extra de-chlorination step is often required before anything else can be accomplished with the treated water. The ⦁OH molecule can combine to create water. The biggest issues would be with bromate formation and excess peroxide, but these can be dealt with in a well-designed advanced oxidation process system.
Mineralization of organics
AOP can convert the organic materials within the water into stable inorganic compounds like water, carbon dioxide, and salts.
Can treat nearly all organic materials and can remove some heavy metals
The highly reactive nature of ⦁OH means these molecules will attack almost any organic materials without discriminating, and therefore, can remove many different contaminants in one reactor vessel, including reducing a few heavy metals.
Can work for disinfection
Especially when used with UV disinfection, the oxidation power of AOP systems make them capable of acting as a disinfection step for any pathogens that may be present in the water.
No sludge production as with chemical or biological processes
An advanced oxidation process does not treat water and wastewater by transferring pollutants into another phase. Other treatment processes create solids like sludge that need to be filtered out and dealt with separately.
Does not concentrate waste for further treatment
Treatment solutions such as membranes result in increased concentrations of the waste contaminants, since they merely separate clean water from the pollutant compounds. AOP meanwhile directly reacts with the pollutants and reduces them to harmless compounds. This process therefore, decreases their concentrations in the effluent.
Cons
Relatively high capital and operating/maintenance costs
Perhaps the biggest drawback of the AOP process is its costs. The most significant are the operating and maintenance costs from the required energy and chemical reagents to operate the system.
Complex chemistry tailored to specific contaminants
Advanced oxidation processes have several different variants. These variants need to be carefully selected to efficiently treat the water/wastewater in question. This process is also a dosage dependent process, so the appropriate amounts of ⦁OH molecules are formed to achieve the desired level of treatment. Such complex chemistry, will likely need highly skilled engineers to design the system correctly.
Removal of residual peroxide may need to be considered
Advanced oxidation process systems utilizing hydrogen peroxide should be carefully controlled for residual H2O2 as it can have potential negative effects on later treatment steps. This residual hydrogen peroxide may be harmful to humans. However, careful design of the system can prevent excess residual H2O2 and any associated consequences.
Are you considering integrating an advanced oxidation process for your water or wastewater treatment system? Contact Genesis Water Technologies at 1-877-267-3699 or via email at customersupport@genesiswatertech.com for a no cost initial consultation to discuss your application.

