Analyzing Water Quality Parameters When Choosing AOP Systems for Water Treatment
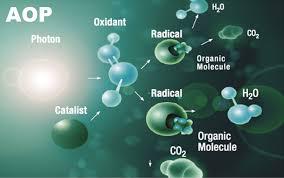
In water and wastewater treatment, chemistry is king. Treatment options are evaluated depending on the quality of water to be treated and the treatment application. Treatment systems including AOP systems, are designed to specifically target certain contaminants and remove or reduce them from the water. This takes places through the power of chemical reactions. Even biological treatments involve chemistry at their core.
Advanced oxidation (AOP) is a water treatment process that utilizes the chemical reaction of oxidation to treat water in the tertiary stage of an integrated water treatment system. The aim is to greatly reduce the micropollutants that escaped from the primary and secondary stages. For certain water qualities, there are certain AOP systems that may be able to treat the pollutants more effectively. Choosing one can be a time consuming process.
In another AOP article, we discuss how to choose the appropriate AOP process for your wastewater treatment applications. One of the key considerations in that decision concerns, both the initial and treated water composition. In that article, we didn’t discuss any specific compounds or conditions that could affect the efficiency of the treatment process, so we are going to evaluate this now.
Below, is a list of some of the key water quality parameters to evaluate when choosing AOP systems for your water treatment application. These can be applied to drinking water or wastewater applications.
Hydroxyl radical scavengers
As the main force behind the AOP process, hydroxyl radicals (⦁OH) need to be produced and maintained at levels that will achieve sufficient treatment.
However, there are some compounds that have an affinity for ⦁OH and will react with these molecules before the target pollutants. This reduces the rate of treatment performance.
Bromide
Bromides are specifically an issue for ozone based AOP systems. Aside from being ⦁OH scavengers, in the presence of ozone, they have the potential to form bromate (BrO3–). Bromate is a known carcinogen. To prevent this, either something must be done to the bromide ions before oxidation or the addition of other chemicals will need to be added to discourage bromate formation.
Natural Organic Matter
Natural organic matter (NOM) is a broad range of dissolved organic materials within the effluent. Like other scavengers, they react with ⦁OH and reduce the reaction rate of the system by using up the radicals needed to oxidize the target pollutants. However, they can also double as blockers for UV light, which can decrease its reaction ability with the oxidizing agent and prevent proper formation of ⦁OH radicals.
Carbonates/Bicarbonates
These compounds are compounds that react with ⦁OH radicals and produce other oxidizing agents, creating a chain of oxidation reactions that reduce the reaction rate and efficiency of the Advanced oxidation process.
Nitrite/nitrate
Nitrate (NO3–) can be converted to nitrite (NO2–) in the presence of UV radiation. Therefore, in the case of Advanced oxidation, UV processes can be hindered by the absorption of radiation by nitrate. Thereby, reducing its availability to react with the oxidizing agent. Reduced to nitrite, nitrates become an ⦁OH scavenger that will reduce the reaction efficiency and removal rate of AOP systems.
pH
How acidic or basic the effluent is can determine the overall concentration of the ⦁OH radical. At certain pH levels, there is an increased presence of a few particular compounds that affect the formation and use of hydroxyl radicals. At higher pH values, there are increased instances of carbonates and bicarbonates which, as seen above, are hydroxyl radical scavengers.
The presence of hydroxide and hydrogen peroxide ions are also higher at greater pH levels. These can increase the production of ⦁OH in both ozone and peroxide-based solutions.
Temperature
The effect of temperature on AOP systems has two facets. On one hand, higher temperatures reduce the solubility of ozone, if ozone is the chosen oxidizing agent. On the other hand, increased temperature speeds up the reaction rates.
Solids
Without effective primary and secondary processes, the turbidity of the effluent in the AOP systems could be higher than desired. At higher levels of turbidity, the absorption of UV radiation by the oxidizing compounds is hindered.
Some of these water quality parameters directly affect the presence and reaction of other parameters. So, there is often a balancing act between adjusting effluent aspects to improve reactivity and efficiency and adjusting them to decrease unwanted constituents.
Some of the hydroxyl scavengers can be dealt with by changing pH levels or temperature, but that may affect the efficiency of the process. Their reduction of reaction rates can also be hindered by introducing other chemicals to intercept them before they come into contact with the ⦁OH radical.
There are many nuances and caveats to choosing AOP systems based on water quality parameters, and it can be difficult to make sense of it when seeing it for the first time.
This is a case where experience comes in handy. The decision can be made much more easily with the aid of an experienced water treatment engineer working alongside of you.
Are you looking for an experienced water treatment firm to help you select, design, and integrate AOP systems based on your water/wastewater quality and application?
Contact Genesis Water Technologies at 1-877-267-3699 or via email at customersupport@genesiswatertech.com for a no cost initial consultation to discuss your application.

